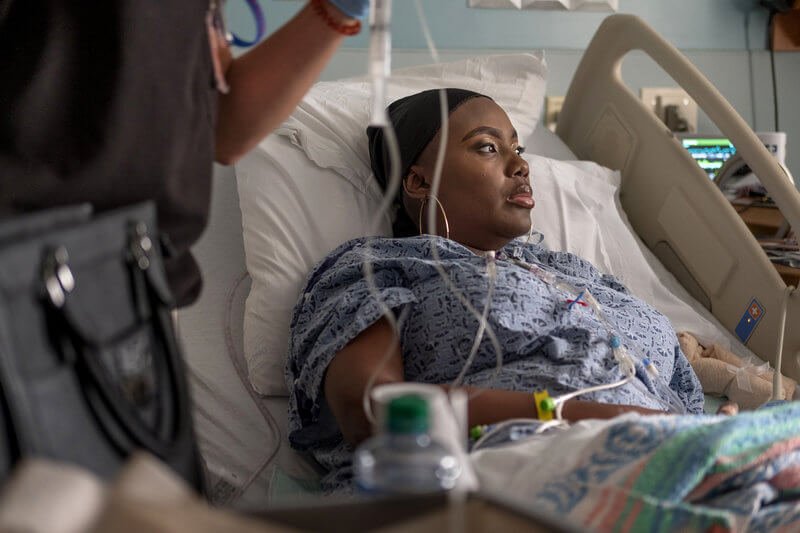
The first new sickle-cell treatment in 20 years will help keep thousands of people out of hospital over the next three years, NHS England has said.
Sickle-cell disease is incurable and affects 15,000 people in the UK and the National Institute for Health and Care Excellence said the hope of reducing health inequalities for Black people, who are predominantly affected and often have poorer health to start with, made the drug worth recommending.
The drug, crizanlizumab, made by Novartis, is injected into a vein and can be taken on its own or alongside standard treatment and regular blood transfusions. And in a trial, patients taking the crizanlizumab had a sickle-cell crisis 1.6 times a year on average, compared with nearly three times a year normally.
The painful episodes, which can require hospital treatment leading to other health issues, are caused by sickle-shaped red blood cells blocking the small blood vessels. But because the trial was small and lasted only a year, it remains unknown how long the benefits last for - and that makes it difficult to judge how cost-effective crizanlizumab is.
Nevertheless, NICE, which recommends treatments in England and Wales, is recommending its use for over-16s, albeit under a special arrangement rather than routinely, on the NHS. And additional data on the treatment will be collected through clinical trials.
The charity Sickle Cell Society said the new treatment brought "new hope" for people living with the world's most common genetic blood condition.
Meindert Boysen, deputy chief executive and director of the Centre for Health Technology Evaluation, at NICE, said: "Often treatment for sickle-cell disease has been limited for years and there has been a lack of treatments for patients whose lives are affected by the condition. Crizanlizuma has shown the potential to improve hundreds of lives and we are delighted to be able to recommend it as the first new treatment for sickle cell disease in two decades."